Oligochaete Community Structure in Paddy fields and Channels in Kole paddy fields, Vembanad Kole wetland, India. 
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2015, Vol. 5, No. 51 doi: 10.5376/ijms.2015.05.0051
Received: 06 Jul., 2015 Accepted: 07 Aug., 2015 Published: 07 Sep., 2015
Vineetha S., Bijoy Nandan S. and Rakhi Gopalan K.P., 2015, Oligochaete Community Structure in Paddy fields and Channels in Kole paddy fields, Vembanad Kole wetland, India, International Journal of Marine Science, 5(51): 1-10 (doi: 10.5376/ijms.2015.05.0051)
Benthic invertebrates including oligochaetes are important components of paddy field fertility due to its significant role in organic matter decomposition and nutrient translocation, but have been studied less. The oligochaete community structure in paddy fields and channels/agricultural ditches of Kole wetlands (a part of Vemband Kole wetlands, a Ramsar site on the south west coast of India) was studied during Punja (summer crop season- January to May 2011). The benthic fauna belonged to the phyla Mollusca, Annelida and Arthropoda. Fifteen oligochaete species of family Naididae, Tubificidae and Lumbriculidae were identified. Seven taxa; Aulodrilus pluriseta, Aulodrilus sp., Aulodrilus piguiti, Branchiodrilus semperi, Stephensonia trivandriana, Pristinella minuta and Pristinella jenkinae were present both in the paddy fields and channels. Six species; Allonais gwaliorensis, Allonais paraguensis, Dero zeylanica, Pristinella accuminata, Pristina menoni, Lumbriculus variegates were exclusively found in the channels and two taxa; Branchiodrilus hortensis, Homochaeta sp. exclusively in the paddy fields. ANOSIM results revealed a similarity in oligochaete composition among paddy fields and channels (Global R=0.19, p>0.05); the lack of environmental specialization in aquatic oligochaetes could be a reason for this. Abundance significantly varied among them, a lower abundance observed in paddy fields (102±161 ind/m2) than channels (891±1409 ind/m2) (ANOVA F1, 24=6.02, p<0.05). Less habitable area in paddy field due to the compartmented substratum by paddy root structures resulted in a reduced abundance in paddy fields. Species richness and diversity showed no significant variation but evenness showed a significant variation between paddy fields and channels (ANOVA F1, 8=10.40, p<0.05). As oligochaetes are characterized by less dispersal and migration ability evenness component would have been more sensitive than other diversity indices.
Introduction
Wetlands are among the most productive, diverse and ecologically sensitive ecosystems on Earth (Ghermandi et al., 2008). Paddy fields are considered as man managed temporary wetlands (Lupi et al., 2013). Rice had been a major source of food for people since 2500 B.C. and paddy fields existed since the beginning of organized agriculture (Edirisinghe et al., 2006). Recently researchers opined that paddy fields could surrogate the loss of natural wetlands due to its biological diversity (Nathuhara, 2013).
Aquatic invertebrates are considered as key components of paddy field fertility due to their significant role in organic matter decomposition and nutrient translocation (Roger et al., 1987). Oligochaetes are known to be a major component of the invertebrate fauna of flooded paddy soils. Oligochaetes especially tubificid worms increase bio available nutrients (nitrogen and phosphorus) in submerged paddy soils, the release rates being proportional to their densities (Roger et al., 1987; Ito and Hara, 2010). They promote nutrient mineralization and suppress weed germination under laboratory conditions (Kikuchi and Kurihara, 1977). Despite their recognized contribution in maintaining soil fertility in wetland paddy fields, little is known about the densities, distribution dynamics, composition, and ecology of field populations. Moreover, paddy fields are not isolated but they are connected by channels or drainage ditches which are small, shallow, line shaped water bodies providing water for agricultural drainage (Verdonschot et al., 2012). Recent studies pointed out the importance of channels as reservoirs of invertebrate biodiversity in agricultural areas emphasizing their role in providing a holistic picture of the ecosystem (Armitage et al., 2003; Herzon and Helenius, 2008).
This study analyzed the oligochaete community structure in paddy fields and channels in Kole paddy fields which are part of Vembanad kole wetlands, a Ramsar site. Kole wetlands are among the water-logged, paddy cultivating areas in Kerala such as Kuttanad (in Alappuzha, Kottayam and Pathanamthitta), Pokkali (in Alappuzha, Ernakulam and Thrissur) and Kaipad (in Kozhikode and Kannur) (Jayan and Sathyanathan, 2010). Kole wetlands were under rice cultivation for the past 200 years since the erstwhile Maharaja permitted to convert this wetland into paddy fields in the early 18th century (Anon., 1989). The cyclical nutrient recharging of the wetland during the flood season made the area as one of the most fertile soils of Kerala. Further they are noted for its high rice production, even the term Kole in Malayalam (the regional language in Kerala, India) means ‘bumper yield of high returns in case flood does not damage the crops’ (Johnkutty and Venugopal, 1993). Though India stands first in area under rice cultivation, second in rice production and has an agricultural based economy (Balachandran 2007), the information on oligochaete community in the paddy fields is limited from India except the works by Ojha et al. (2010) and Hegde and Sreepada (2014).
Materials and Methods
Study area
The study area is a part of the Ponnani Kole lying between Maranchery and Veliyamkodu in Malappuram district (100 72’N 750 98’E) (Figure 1). The Kole lands covering an area of 13,632 ha. spread over Thrissur and Malappuram districts of Kerala extending from northern bank of Chalakkudy river in the South to the southern bank of Bharathappuzha river in the North. The Viyyam dam is situated at the downstream of end of Kole lands which prevents the intrusion of salt water to the paddy fields. The Kole lands are believed to be lagoons formed by the recession of the seas centuries back. A shallow portion of the sea along the western periphery of the main land was isolated and they were gradually silted up during rains making the lagoons shallow. The farmers then bunded the fields, dewatered and raised paddy in summer months. During the rains, the inflow into the basin submerges all the kole areas. The area normally remains flooded from June to January. Normally two rice crops are raised in Kole wetlands, a summer crop (punja in December/January- April/May) and an additional crop (Kadumkrishi) (Raj and Azeez, 2009). The main crop is Punja (Summer crop). Towards the end of the north east monsoon (October to December), water from the paddy fields are pumped out and sowing or transplanting is done by January. Dewatering is done by centrifugal pumps or petti and para which is an indigenous pumping device. The kole lands are dewatered after protecting the paddy fields (Padavu or Padashekharam) with permanent or temporary earthen bunds (Mattoms) (Johnkutty and Venugopal, 1993). The crop is harvested in May, soon after which the field gets flooded due to the South West Monsoon (June to September).
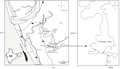 Figure 1 Location of sampling sites in Maranchery Kole wetlands, P refers to paddy fields and C refers to channels |
Sampling procedure and methods
A total of five sampling stations were chosen, three stations were paddy fields and two were channels. Channels and paddy fields were separated by an earthen bund. The field sampling was carried out for a complete crop season ‘punja’ extending from January to May 2011 on a monthly basis for the study of oligochaetes and environmental parameters.
As the water body was shallow (average depth 0.71 m), water samples were collected using a locally fabricated shallow water sampler of 1 litre capacity. The samples were stored in plastic containers and kept frozen till analysis. The sediment samples for the analysis were collected using a Van Veen grab of size 0.45m2. Temperature of the water and sediment samples were measured in the field using a standard degree centigrade thermometer of 0°C to 50°C range and 0.l°C accuracy. pH was measured using Systronics digital pH meter model MK VI. Dissolved oxygen was analyzed by modified Winkler method (Strickland and Parsons, 1972). Organic carbon was determined by Walkley-Black method then converted to organic matter by multiplying with Van Bemmelen factor of 1.742 (Jackson, 1973). Particle size was analyzed using particle analyzer Sympatrec T 100 laser diffraction granulometer, made in Germany.
Sediment samples in replicate were collected for the analysis of macrobenthos using a Van Veen grab of size 0.45m2. The samples were washed in the field itself through a sieve of mesh size 500 μm and those that are retained in the sieve were collected and preserved in 5% formalin (Holme and McIntyre 1971; Eleftheriou and McIntyre, 2005). The organisms were separated into different taxonomic groups (oligochaetes, insects, gastropods and hirudinea). Oligochaetes were identified up to species level by temporarily mounting the specimens using Amman’s Lactophenol (Phenol, Lactic acid, Glycerol, and water in the ratio of 1:1:2:1) (Brinkhrust and Jamieson, 1971). The taxonomic keys of Brinkhrust and Jamieson (1971) and Naidu (2005) were used for the identification.
Statistical Analysis
One way ANOVA was used to determine the significant difference in numerical abundance of oligochaetes between the paddy fields and channels using SPSS 16.0. Abundance data was fourth root transformed to meet the ANOVA assumptions. The data on oligochaetes was subjected to multivariate and univariate analysis by using the Primer software version 6.0 (Clarke and Gorley, 2006). ANOSIM was used to analyze the similarity of oligochaete assemblages between paddy fields and channels. Non metric multi-dimensional scaling, a hierarchical cluster analysis was used for the pictorial representation of the pattern of oligochaete composition in the paddy fields and channels (relative abundance). Ordinations were based on distance matrices, which were computed using Bray Curtis coefficient. The univariate indices of diversity such as species richness by Margalef’s index (Margalef, 1958), species diversity by Shannon index (Shannon Wiener, 1949) and species evenness by Pielou's index (Pielou, 1966) were calculated for paddy fields and channels separately.
Results
Environmental parameters
The results of the environmental parameters analyzed are given in Table 1. There was no significant difference in environmental parameters analyzed in paddy fields and channels. Depth showed a marked difference between paddy fields (0.31±0.11 m) and channels (1.1±0.28 m). Water and sediment temperature remained lower in the paddy fields. The water pH was slightly acidic in both paddy fields and channels. Sediment pH remained neutral. Organic matter was higher in the paddy fields (6.95±1.34%), than channels (5.48±1.58%). The sediment was clayey silt in paddy fields and sandy silt in channels.
.jpg) Table 1 Mean (±standard deviation) of environmental parameters measured in paddy fields and channels in Maranchery Kole wetlands during Punja crop season January -May 2011 |
Faunal Composition
The macrobenthic fauna recorded from Maranchery Kole wetlands included three phyla (Mollusca, Annelida and Arthropoda) and five classes (Gastropoda, Oligochaeta, Hirudinea, Insecta and Crustacea) (Figure 2).
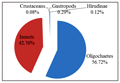 Figure 2 Mean percentage composition of benthic fauna in paddy fields and channels in Maranchery Kole wetlands |
The most abundant benthic class was Oligochaeta (56.72%) followed by Insecta (42.76%) while Gastropoda (0.29%), Hirudinea (0.12%) and Crustacean (0.08%) appeared occasionally with very low abundance. In paddy fields insects (61%) were the most abundant group followed by oligochaetes (37%). Gastropods (1%) and crustaceans (1%) made a single appearance in January 2011. Oligochaetes were the most abundant in channels (60.39%) followed by insects (39.30%). Gastropod (0.15%) and Hirudinae (0.15%) were present in February and April 2011 respectively. A total of fifteen oligochaete species were collected from the samples (Table 2). Among them eleven species belonged to the family naididae, three species belonged to the family tubificidae and one species to the family lumbriculidae. In terms of percentage abundance, the species of the family naididae had the highest contribution (56%) followed by tubificidae (43%) and lumbriculidae (1%). Seven taxa, Aulodrilus pluriseta, Aulodrilus sp., Aulodrilus piguiti, Branchiodrilus semperi, Stephensonia trivandriana, Pristinella minuta, Pristinella jenkinae were recorded both in the paddy fields and channels. Six species, Allonais gwaliorensis, Allonais paraguensis, Dero zeylanica, Pristinella accuminata, Pristina menoni, Lumbriculus variegates were exclusively found in the channels and two taxa, Branchiodrilus hortensis, Homochaeta sp., exclusively in the paddy fields. Among the species exclusively present in channels, Allonais gwaliorensis, Allonais paraguensis, Dero zeylanica were observed only once in the sample, during an unusual abundance observed in May 2011. The most abundant species in the paddy fields was Aulodrilus sp. (23%) closely followed by Aulodrilus pluriseta (20%), whereas Aulodrilus pluriseta (49%) was the most abundant in the channels. The analysis of oligochaete community composition using ANOSIM revealed the absence of a significant difference in oligochaete composition between the paddy fields and channels (Global R=0.19, p> 0.05).
.jpg) Table 2 List of oligochaete species in paddy fields and channels of Maranchery Kole wetlands. + = species present - = species absent |
Pattern of faunal abundance
The monthly variation in mean numerical abundance of oligochaetes showed a slight zigzag pattern throughout the crop season, decreasing from January 2011 (14%) to February (6%), increasing in March (8%), again decreasing in April (4%) and showed a sharp increase in May 2011 (68%) (Figure 3). When the mean numerical abundance of oligochaetes in the paddy fields was considered, the maximum abundance was observed in March 2011 (36%) and minimum in February (6%). The channels showed the minimum abundance in April 2011 (3%). In May 2011, an unusually high abundance of 71% (4355 ind./m2) was observed in channels. There existed a significant difference in numerical abundance of oligochaetes among the paddy fields and channels (ANOVA F1, 24=6.02, p<0.05). Abundance of oligochaetes was high in the channels than the paddy fields throughout the crop season.
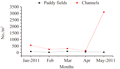 Figure 3 Monthly mean variation in oligochaete abundance in paddy fields and channels in Maranchery Kole wetlands |
Oligochaete community structure
Though a significant difference in numerical abundance was observed between the paddy fields and the channels, the species diversity (Shannon wiener index) and richness (Margalef’s index) showed no significant variation among them. Evenness (Pielou's index) showed a significant variation (ANOVA, F1, 8=10.40, p<0.05). A higher diversity was recorded in channels (mean=1.88±0.34) than paddy fields (mean=1.65±0.68). The highest diversity value was observed in January 2011 (2.41), the beginning of the crop season in the paddy fields and lowest in May 2011 (0.91). On the contrary, in the channels, the highest diversity was in May 2011 (2.42), the end of the crop season and minimum in February 2011 (1.53) (Figure 4). Though in paddy fields, the maximum and minimum diversity was recorded in the beginning and end of the crop season respectively, no linear declining pattern in diversity value was apparent. Channels showed an increasing trend in diversity from the beginning (1.73 in January) to end of the crop season (2.23 in May 2011) except a very slight decline in diversity in February and April (1.88). Margalaf’s species richness also was higher in channels (mean=0.84±0.25) against paddy fields (mean=0.70±0.36) (Figure 5). However there existed a significant variation in evenness between paddy fields and channels, it showed a higher value in paddy fields (mean=0.93±0.05) than channels (mean=0.73±0.12) (Figure 6). Evenness was maximum in February (1.00) in paddy fields and the minimum evenness was observed in March (0.37). In channels, evenness was maximum in April (0.94) and minimum in February (0.59). In paddy fields, species richness followed the similar trend as diversity, showed maximum and minimum values in January (1.14) and April (0.32) respectively. In channels the maximum and minimum values were in April (1.23) and May (0.60) respectively.
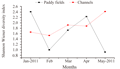 Figure 4 Monthly variation in oligochaete diversity paddy fields and channels in Maranchery Kole wetlands |
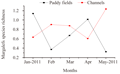 Figure 5 Monthly variation in oligochaete richness in paddy fields and channels in Maranchery Kole wetlands |
The distance between data points on nMDS plot showed no clear delineation of data points between paddy fields and channels (i.e., data points were more close) however a clear separation in some months especially in May and February 2011 (i.e., data points were more dispersed) was apparent (Figure 7). The appearance of the species, Allonais gwaliorensis, Allonais paraguensis and Dero zeylanica exclusively in the channels due to the increased abundance in May 2011 caused the separation of the channel from the rest of the group in May 2011 in the nMDS ordination plot. The low abundance and the absence of Aulodrilus pluriseta in the sample caused the delineation of the paddy fields in May 2011 whereas in February 2011, the absence of Aulodrilus pluriseta and Aulodrilus sp. lead to the separation in the nMDS ordination plot.
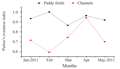 Figure 6 Monthly variation in oligochaete evenness in paddy fields and channels in Maranchery Kole wetlands |
 Figure 7 Two dimensional nMDS ordination of oligochaete community structure in paddy fields and channels in Maranchery Kole wetlands |
Relationship between biotic and environmental patterns
BIOENV results for the paddy fields and channels are given in (Table 3). The results revealed a moderate correlation with the combination of a few environmental parameters. Dissolved oxygen and the percentage of clay were the BEST environmental variables explaining the variability of oligochaetes in paddy fields (r=0.29) whereas dissolved oxygen, the percentage of clay and the percentage of silt were the important variable in channels. (r=0.28).
.jpg) Table 3 BIOENV results showing the spearman correlation coefficient (ρ) for correlation with oligochaete abundance in paddy fields and channels in Maranchery Kole wetlands |
Discussion
Faunal Composition
Though paddy fields and channels differed in the physical structure, there was no significant difference in environmental parameters between them. A lower water and sediment temperature in the paddy fields could be due to the shading by paddy plants. Higher organic matter in paddy fields could be due to the accumulation of paddy leaves also a higher water level might have reduced the organic matter in channels due to dilution as observed by Ali et al. (2002).
Paddy fields were characterized by emergent vegetation, reduced water levels, compartmentalized benthic habitat whereas channels were line shaped, deeper open water habitats with submerged vegetation. In spite of these differences, the oligochaete composition did not show a significant difference between them. The less prominent niche specialization of oligochaetes proved by many studies could be a reason for this (Verdonschot and Higler, 1989; Verdonschot, 1999). The paddy fields and the channels explored in this study were very close to each other, separated by an earthen bund, also until December 2010, both paddy fields and channels were submerged thus were connected to each other and behaved as the same water body. Then the preparation for paddy cultivation was carried out, which separated the area into paddy fields and channels. Though the practices prior to farming such as draining, ploughing etc. would have disturbed the area, oligochaetes are known to survive the unfavorable period by mechanisms such as diapausing eggs; resistant cysts enclosing young, adults or fragments of individuals (Williams, 1987). The oligochaetes prevailed in the flooded period would have survived in the area as dormant structures during unfavorable period and became active once there was sufficient water, which could be the probable reason for the similar oligochaete composition in the paddy fields and the channels.
Similar studies across the world revealed different results on oligochaete composition of paddy fields. In Philippines the oligochaete populations were dominated by Limnodrilus hoffmeisteri and Branchiodrilus sowerbyi, of the family tubificidae, the species belonging to naidids, lumbriculids, and enchytraids were also represented (Simpson et al., 1993). Heckman (1974) and (1979) recorded oligochaete species exclusively of the family naididae from the paddy fields from Laos and Thailand respectively. According to Senapati et al., (1991), the earthworm Darwida willsi dominated the paddy fields in India. The families aeolosomatidae, tubificidae and megascolecidae constituted oligochaete fauna in Chapra, Bihar (Ojha et al., 2010). A recent study from the paddy fields of Dakshin Kannada revealed a very high density of Aulophorus furcata of the family naididae (Hegde and Sreepada, 2014).
Pattern of faunal abundance
The significant variation numerical abundance of oligochaetes between paddy fields and channels could be explained by the area of available habitat. The increase in habitable area could result in an increase in the number of organisms (Sommer and Horwitz, 2009). Unlike the bottom of the channel, bottom of the paddy field was compartmented by paddy root structures providing insufficient space for the proper development of benthic fauna, could be a probable reason as observed by Ojha et al., (2010). In this study, on an average, the abundance of oligochaetes in paddy fields was 8.7 times less than that of channels; a similar report was from Chapra, Bihar where the total average number of benthic macrofauna of paddy fields was nearly eight times less in contrast to the benthos from adjacent ponds (Ojha et al., 2010).
There was an unusually high abundance of oligochaetes in May 2011 in the channels. The unusually high benthic abundance was observed previously by Wishner et al. (1990), the enriched sediment resulting from reduced consumption and degradation of sinking material which supply high food level, was the suggested reason. According to Brinkhurst (1996), the unusual abundance of oligochaetes especially tubificids were clear indication of excess organic matter in an environment where oxygen deficiency and high silt loads combine to kill most of the fauna. But in this study, in May 2011, all the environmental parameters analyzed, especially oxygen, organic matter and silt content remained similar to the other samples. Further though tubificids dominated (61%), naidids also showed a good abundance (39%) in the particular samples. Along with oligochaetes in the benthic sample, insect larvae especially chironomids also showed an unusually high abundance comparable to that of oligochaetes. Both Wishner et al., (1990) and Brinkhurst (1996) emphasized the significance of the abundance of food source for the unusual benthic abundance. The organic matter in the present study was higher throughout the study period, ensuring a food source for benthos. Apart from the quantity of organic matter, the nutritional quality is also important in determining benthic abundance (Neira 2011). The abundance of good quality food would have favored the unusual benthic abundance in May 2011. Or some specific, localized condition would have resulted in the patchy distribution of the fauna in channels as suggested by Verdonschot et al., (2011).
The average numerical abundance of oligochaetes was less in this study when compared to similar studies. Roger (1996) and Simson et al., (1993) recorded tubificid populations of about 10,000 and 40,000 ind.m-2 in Japan and Philippines paddy fields respectively. Ojha et al., (2010) observed 178 ind.m-2 from Chapra, Bihar. Along with the difference in zoogeographic factors, the difference in sampling methods also would have contributed to the difference.
Community structure
There were two contradictory possibilities about the presence of oligochaetes in the paddy fields. The reduced habitable area due to paddy plantation would have reduced the number of oligochaetes in paddy fields. On the contrary, due to the same reason the oligochaetes would have utilized the available habitable open areas as a refuge, thus ensuring a fairly high diversity and richness in areas free from paddy roots. This could be viewed analogous to the increased benthic richness observed in available water patches, when the rest of the substratum are dry, due to the use of the water patches as refuge by the benthic organisms (Sommer and Horwitz 2009). Though the numerical abundance varied significantly between channels and paddy fields, comparable species richness and diversity was maintained throughout the study period in the paddy fields which could be due to the usage of the available habitable area by the oligochaetes in the restricted habitat. Though the species number in the paddy fields was less than that of the channels, the species diversity and richness was comparable among them. A study on benthic invertebrates in paddy fields and channels in Italy highlighted significant differences between them, with higher diversity observed in channels (Lupi et al., 2013).
Though the univariate indices such as richness, species number and diversity showed no significant variation between paddy fields and channels, evenness varied significantly between paddy and channel. According to Ma (2005), the individual diversity components were found to respond independently to different ecological factors. When the taxa and the studied system was regulated more by dispersal and migration, species richness was found to be more sensitive. Oligochaetes are characterized by the absence of a planktonic dispersal stage which limits their dispersal, also as they are benthic crawlers, their migration ability is also limited (Wiggins et al., 1980, Levin et al., 1996) so the sensitivity of the richness component would be limited. On the other hand, evenness was found to be more sensitive to biotic interactions (Ma 2005). Although biotic interactions have not been analyzed in this study, previous studies have proved that in the temporary wetlands, the biotic interactions are important in structuring the communities (Peltzer and Lajmanovich, 2004). Moreover, at small and large spatial scales the importance of biotic interactions in structuring communities have been emphasized (Hildrew et al., 2004).
The number of oligochaete species recorded in this study was more compared to similar studies from India. A similar study from Chapra, Bihar revealed 4 oligochaete species (Ojha et al., 2010), whereas from Dakshin Kannada revealed 3 oligochaete species (Hegde and Sreepada, 2014).
Relationship between biotic and environmental patterns
The present study shows the importance of substrate composition and dissolved oxygen in determining the oligochaete pattern irrespective of paddy fields and channels. The importance of substrate composition in determining the oligochaete pattern in water bodies was documented in previous studies by Stimpson et al., (1975). The present study showed that dissolved oxygen was a major factor structuring oligochaete communities. Generally, some pollution tolerant oligochaete species such as L. hoffmeisteri, Tubifex tubifex and Bothrioneurum vejdovskyanum were reported to be able to tolerate extremely oxygen-deficient environments (Brinkhurst, 1974), but they were absent in Maranchery paddy fields.
Various studies revealed a variety of factors associated with oligochaete distribution and abundance in water bodies including substrate composition (Stimpson et al., 1975), organic matter content (Lazim and Learner, 1987) sediment chemistry (Sarkka and Paasivirta, 1972) and fish predation (Kajak and Dusoge, 1971). Previous studied showed that in paddy fields, the species density showed positive correlation with nitrogen, phosphorous and potassium. Further oligochaetes were scarce or absent where algae were scarce, pesticides and fertilizers were applied and farm manure was not applied (Hedge and Sreepade, 2014).
Some factors which we have not directly measured also would have played a major role in the distribution of benthic fauna like the area of the habitable patch available without paddy roots for the survival of oligochaetes. The significance of the area of the habitable patch in determining the benthic structure was proved in previous studies (Fleishman et al., 2002). Also predation by chironomids on oligochaetes especially from the chironomids belonging to the genus Tanypus, Procladius and Ablabesmyia were reported (Loden, 1973; Arslan and Sahin, 2006). The paddy fields were characterized by the abundance of chironomids, this also would have played a key role in determining the community structure.
The present study showed that irrespective of the habitat alterations associated with the paddy cultivation, the oligochaetes community remained healthy in Kole fields implying its potential to harbor high biodiversity. Most importantly, there was no usage of agrochemicals during the study period, but further researches are needed to evaluate its impacts. Traditionally, the research on aquatic systems was focused on permanent waters thereby ignoring temporary waters like paddy fields which resulted in fewer data bases of these unique habitats. Research on oligochaetes in the paddy fields is significant when the ill effects of chemical fertilizers and pesticides are revealed gradually, since oligochaetes are known to improve soil fertility and reduce weed formation. Such baseline information from the field is essential for management and conservation aspects.
Authors contribution
S. Bijoy Nandan is the supervising guide, Rakhi Gopalan K.P made contribution in sampling and analysis.
Acknowledgements
The authors are thankful to the Head of the Department of Marine biology, Microbiology and Biochemistry, Cochin university of Science and Technology for providing necessary facilities. This study was a part of the research project funded by Kerala state Biodiversity Board, the authors are thankful to them. First author is thankful to University Grants Commission for the research fellowship.
References
Ali A., Frouz J., and Lobinske R.J., 2002. Spatiotemporal effects of selected physico-chemical variables of water, algae and sediment chemistry on the larval community of nuisance Chironomidae (Diptera) in a natural and a manmade lake in central Florida. Hydrobiologia 470: 181-193
http://dx.doi.org/10.1023/A:1015696615939
Ali A.B., 1998. Paddy agroecosystem and the maintenance of biodiversity. p. 25-36. In Nashriyak BM, Ho NK, Ismael BS, Ali AB, Lun KY (eds.) Paddy agroecosystem of the Muda Irrigation Scheme, Malaysia. Mintmada Malaysia, 250 pp.
Anonymous., 1989. Scheme for studying the possible changes in the ecosystem consequent on the conservation of Thannermukkam Bund, Thrissur, Kerala. Kerala Agricultural University.
Armitage P.D., Szoszkiewicz K., Blackburn J.H., and Nesbitt I., 2003. Ditch communities: a major contributor to floodplain biodiversity. Aquatic Conservation: Marine and Freshwater Ecosystems 13, 165-185DOI: 10.1002/aqc.549
http://dx.doi.org/10.1002/aqc.549
Arslan N., and SahinY.Y., 2006. A preliminary study on the identification of the littoral oligochaete (Annelida) and chironomidae (Diptera) fauna of Lake Kovada, a national park in Turkey. Turk J Zool 30:67-72
Bahaar S.W.N., and Bhat G.A., 2011. Aquatic biodiversity in paddy fields of Kashmir Valley (J and K) India. Asian Journal of agricultural research 5(5):269-276, doi:10.3923/ajar.2011.269.276
http://dx.doi.org/10.3923/ajar.2011.269.276
Balachandran P.V., 2007. Rice scenario of Kerala and the future strategies. Proceedings of XIX Kerala Science Congress, Kannur, Kerala 22-32 pp.
Brinkhurst R.O., and Kennedy C.R., 1965. Studies on the biology of the Tubificidae (Annelida, Oligochaeta) in a polluted stream. J Anim Ecol., 34:429-443
http://dx.doi.org/10.2307/2659
Brinkhurst R.O., and Jamieson B.G.M., 1971. Aquatic Oligochaeta of the World. Oliver and Boyd, Edinburgh.
Brinkhurst, R.O., 1972. The role of sludge worms in eutrophication US EPA, Washington D.C, Ecol. Res.ser.E.P.R3-72-004 Aug 1972
Brinkhurst, R.O., 1996, On the role of tubificid oligochaetes in relation to fish disease with special reference to the Myxozoa: Annual Review of Fish Diseases, v. 6, p. 29-40.
Clarke K.R., and Gorley R.N., 2006. PRIMER v6: User Manual/Tutorial. PRIMER-E: Plymouth, UK, 91pp.
Edirisinghe J.P., and Bambaradeniya C.N.B., 2006. Rice fields:an ecosystem rich in biodiversity. Journal of national science foundation Srilanka 34 (2):57-59
Eleftheriou A., and McIntyre A.D., 2005. Methods for the study of marine benthos, Blackwell Science, 418 pp.
http://dx.doi.org/10.1002/9780470995129
Fleishman E., Ray C., Sjogren-Gulve P., Boggs C.L., and Murphy D.D., 2002. Assessing the roles of patch quality, area and isolation in predicting metapopulation dynamics. Conserv Biol 16:706-716
http://dx.doi.org/10.1046/j.1523-1739.2002.00539.x
Ghermandi A, Van den Bergh J.C.J.M., Brander L.M., de Groot H.L.F., Nunes P.A.L.D.,2010. Values of natural and human-made wetlands: a meta-analysis. Water Resour. Res., 46 (12) pp. 1-12
Heckman C.W., 1974. The seasonal succession of species in a rice paddy in Vientienne, Laos. Int Rev Ces Hydrobiol 59489-507
Heckman C.W., 1979. Rice field ecology in northeastern Thailand. The effect of wet and dry seasons on a cultivated aquatic ecosystem. Monographiae Biologicae, vol 34, W Junk, The Hague International Rice Research Institute (1985) Annual report. IRRI, Manila, Philippines.
Herzon I., and Helenius J., 2008. Agricultural drainage ditches, their biological importance and functioning. Biological Conservation 141, 1171-1183
http://dx.doi.org/10.1016/j.biocon.2008.03.005
Holme N.A., and Mc Intyre A.D., 1971. Methods for study of Marine Benthos, IBP Hand book No.6, Blackwell Scientific Publications.
Ito T, Hara K. 2010. Impact of Tubificid Worm on Nutrient Dynamics in Paddy Field. J.F.S., 7: 47-50.
Jackson M.L. 1973. Soil chemical analysis, Printers-Hall India Ltd, New Delhi.
Johnkutty I., and Venugopal V.K., 1993. Kole wetlands of Kerala, Kerala Agricultural University Thrissur 68pp.
Kajak Z., and Dusoge K., 1971. The regularities of vertical distribution of benthos in bottom sediments of three Masurian lakes. Ecol. Pol. 19: 485-499
Kikuchi E., Furusaka C., and Kurihara Y., 1975. Survey of the fauna and flora in the water and soil of paddy fields. Rep Inst Agr Res Tohoku Univ 26:25-35
Kikuchi E., and Kurihara Y., 1977. In vitro studies on the effects of tubificids on the biological, chemical and physical characteristics of submerged ricefield soil and overlying water. Oikos 26, 348-356
http://dx.doi.org/10.2307/3543626
Kurihara Y., 1989. Ecology of some ricefields in Japan as exemplified by some benthic fauna, with notes on management. Int Rev Ges Hydrobiol 74507-548
Lazim M.N., and Learner M.A., 1987. The influence of sediment composition and leaf litter on the distribution of tubificid worms (Oligochaeta). A field and laboratory study. Oceologia 72: 131-136
http://dx.doi.org/10.1007/BF00385057
Levin L.A., Talley D., and Thayer G., 1996. Succession of macrobenthos in a created salt marsh. Marine Ecology Progress Series 141:67-82
http://dx.doi.org/10.3354/meps141067
Loden Michael S. 1974. Predation by chironomid (Diptera) larvae on oligochaetes, Limnology and Oceanography.
Lupi D., Rocco A., and Rossaro B., 2013. Benthic macroinvertebrates in Italian rice fields J. Limnol 72(1):184-200.doi 10.4081/jlimnol.2013.e15
http://dx.doi.org/10.4081/jlimnol.2013.e15
Ma M., 2005. Species richness vs. evenness: independent relationship and different responses to edaphic factors. Oikos 111:192-198
http://dx.doi.org/10.1111/j.0030-1299.2005.13049.x
Margalef D.R., 1958. Information theory in ecology. Gen. Syst., 3, 36-71.
Naidu V.K., 2005. Fauna of India and adjacent countries. Zoological Survey of India Publication.
Nathuhara Y. 2013. Ecosystem services by paddy fields as substitutes of natural wetlands in Japan. Ecological engineering 56:97-106.
Neira C, Sellanes J, Levin LA, Arntz WE. 2001. Meiofaunal distributions on the Peru margin: relationship to oxygen and organic matter availability. Deep-Sea Research. Pt. I: Oceanography . Research Paper 48:2453–2472.
Ojha N.K., Pandey M.K., and Yadav R.N., 2010. Studies on macrobenthic community of paddy field of Rural Chapra. Bioscan: Special issue, Vol. 2: 579-585; ISSN: 0973-7049
Peltzer P.M., and Lajmanovich R.C., 2004. Anuran tadpole assemblages in riparian areas of Parana´ River (Argentina).Biodiversity and Conservation 13(10)1833-1842
http://dx.doi.org/10.1023/B:BIOC.0000035870.36495.fc
Pielou E.C., 1966. Shannon's formula as a measurement of specific diversity and its use and misuse. American Naturalist 100(914):463-465
http://dx.doi.org/10.1086/282439
Hegde P.R., and Sreepada K.S., 2014. Reports on aquatic oligochaetes (Naididae) in paddy fields of Moodabidri Taluk, Dakshina Kannada, South India. Journal of entomology and zoology studies; 2 (2): 101-107 ISSN 2320-7078
Hildrew A.G.,. Woodward G, Winterbottom J.H., Orton S.2004.Strong density dependence in a predatory insect: large scale experiments in a stream. Journal of Animal Ecology, 73,pp. 448–458.
Jayan PR, and Sathyanathan N., 2010. Overview of farming practices in the water-logged areas of Kerala, India. International Journal of Agriculture and Biological Engineering 3(41).
Raj P.P.N., and Azeez P.A., 2009. Real estate and agricultural wetlands in Kerala. Economic and Political Weekly January 31, 63-66
Roger P.A., Grant I.F., Reddy P.M., and Watanabe I. 1987. The photosynthetic aquatic biomass in wetland rice fields and its effect on nitrogen dynamics. Efficiency of nitrogen fertilizers for rice. International Rice Research Institute, Los Baños, Philippines, 43-68 pp.
Roger P.A., and Kurihara Y., 1988. Floodwater biology of tropical wetland ricefields. In: Int Symp Paddy Soil Fertility. International Soil Science Society, The Hague, 275 -300 pp.
Sarkka J., and Paasivirta L., 1972. Vertical distribution and abundance of macro- and micro-fauna in the profundal sediments of Lake Paijanne, Finland. Ann. Zool. Fenn. 9: 1-9
Senapati B.K., Biswal J., Sahu S.K., and Pani S.C., 1991. Impact of malathion on Darwidu willsi Michaelsen, a dominant earthworm in paddy fields. Pedobiologia 35:117-128
Shannon C.E., and Weaver W., 1949. The Mathematical theory of communication. University of llinois Press, Urbana, Illinois 117 pp.
Simpson E.H., 1949. Measurement and diversity. Nature 163:688
http://dx.doi.org/10.1038/163688a0
Simpson I.C., Roger P.A., Oficial R., and Grant I.F., 1993. Density and composition of aquatic oligochaete populations in different farmers paddy fields Biol fertile Soils 16:34-40
Simpson I.C., Roger P.A., Oficial R., and Grant I.F., 1993. Impacts of agricultural practices on aquatic oligochaete populations in paddyfields. Biol Fertil Soils 1627-33
Sommer B., and Horwitz P., 2009. Macroinvertebrate cycles of decline and recovery in Swan Coastal Plain (Western Australia) wetlands affected by drought-induced acidification Hydrobiologia 624:191–203 doi 10.1007/s10750-008-9692-6
http://dx.doi.org/10.1007/s10750-008-9692-6
Sreenivasan J.T. 2011. Agriculture- wetland interactions: How to sustain rice production in the Kole land, Kerala. Research unit for livelihoods and natural resources, Centre for economic and social studies. Policy Brief No.1, January 2011.
Stimpson K.S., Brice J.R., Barbour M.T., and Howe P., 1975. Distribution and abundance of inshore oligochaetes in Lake Michigan.Trans. am. microsc. Soc. 94: 384-394
http://dx.doi.org/10.2307/3225503
Strickland J.D.H., and Parsons T.R., 1972. A practical handbook of seawater analysis. Bull Fish Res Bd Can, 2nd Edn vol 167 310pp.
Verdonschot P.F.M., 1999. Micro-distribution of oligochaetes in a soft-bottomed
lowland stream (Elsbeek; The Netherlands) Hydrobiologia 406: 149-163
http://dx.doi.org/10.1023/A:1003796403364
Verdonschot P.F.M., and Higler L.W.G., 1989. Macroinvertebrates in Dutch ditches: a typological characterization and the status of the Demmerik ditches. Aquatic Ecology 23, 135-142
http://dx.doi.org/10.1007/bf02256730
Verdonschot R.C.M., Keizer-Vlek H.E., and Verdonschot P.F.M., 2012. Development of a multimetric index based on macroinvertebrates for drainage ditch networks in agricultural areas. Ecological Indicators 13 232-242 doi:10.1016/j.ecolind.2011.06.007
http://dx.doi.org/10.1016/j.ecolind.2011.06.007
Verdonschot R.C.M., Keizer-Vlek H.E., and Verdonschot P.F.M., 2011. Biodiversity value of agricultural drainage ditches: a comparative analysis of the aquatic invertebrate fauna of ditches and small lakes. Aquatic Conserv: Mar. Freshw. Ecosyst. 21: 715-727
http://dx.doi.org/10.1002/aqc.1220
Wiggins G.B., Mackay R.J., and Smith I.M., 1980. Evolutionary and ecological strategies of animals in annual temporary pools. Archiv fu¨r Hydrobiologie/Supplement 58:97-206
Williams D.D., 1987. The ecology of temporary waters. Croom Helm, London 205pp.
http://dx.doi.org/10.1007/978-94-011-6084-1
Wishner K., Levin L., Gowing M., and Mullineaux L., 1990. Involvement of the oxygen minimum in benthic zonation on a deep seamount. Nature 346: 57-59
http://dx.doi.org/10.1038/346057a0
. PDF(621KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Vineetha Saraswathy
. Bijoy S.
. Rakhi K.P.
Related articles
. Oligochaetes
. Community structure
. Kole wetlands
. Paddy fields
. Channels
Tools
. Email to a friend
. Post a comment


